Executive Certificate in Product Development and Manufacturing of IVD and SaMD

Date duration
Programme Period
18 Nov 2024 to 21 Mar 2025

Delivery mode
Program Description
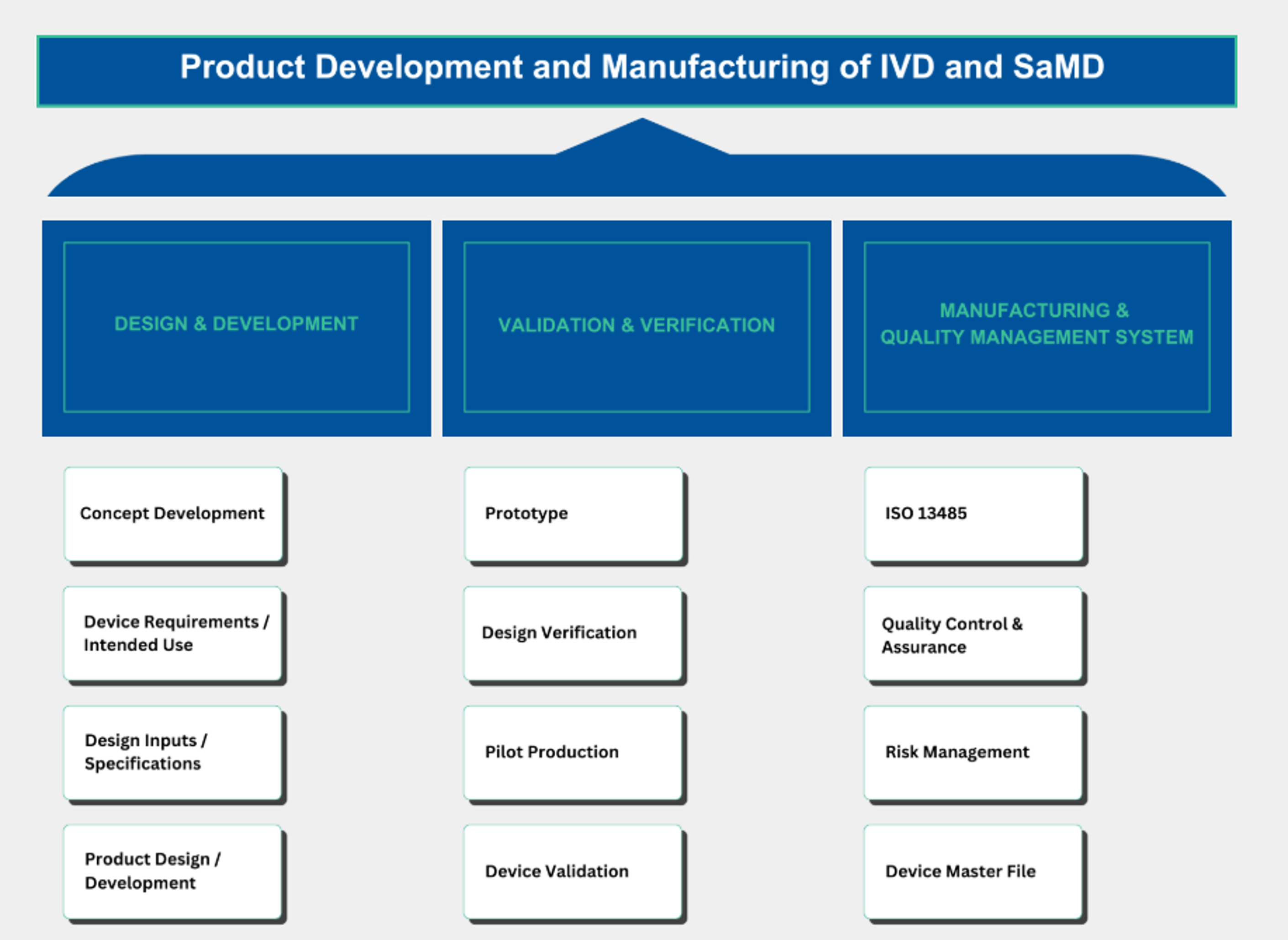
The Executive Certificate in Product Development and Manufacturing of IVD and SaMD programme consists of 3 courses with a total of 4 units.
| Course Title |
|
Design and Development |
|
Validation & Verification |
|
Manufacturing and Quality Management System |
Courses Description
Design and Development
This course will provide you with a fundamental understanding of the principles involved in progressing from product conceptualisation and design to testing and qualification. You will gain the knowledge needed to make informed decisions when planning to transform research ideas into properly developed In Vitro Diagnostic (IVD) devices and Software as a Medical Device (SaMD).
Validation and Verification
This course will help you better understand the regulatory perspectives and standards required for the commercial approval of health products, including risk assessment and mitigation plans. You will gain insights that will support the development, market entry, and effective use of In Vitro Diagnostics (IVDs) and Software as a Medical Device (SaMD).
Manufacturing and Quality Management System
In this workshop, you will hear from experts discussing key manufacturing requirements, including quality management system standards, and the regulatory steps needed for a company to release a product for sale in regulated markets. This workshop will provide valuable insights into navigating the regulatory landscape for product launch.